ocrevus start up form
It May Be Time to Look Into an Option Like KESIMPTA. Date of birth.

Multiple Sclerosis Ms Types Symptoms And Causes
Prescribers first name.

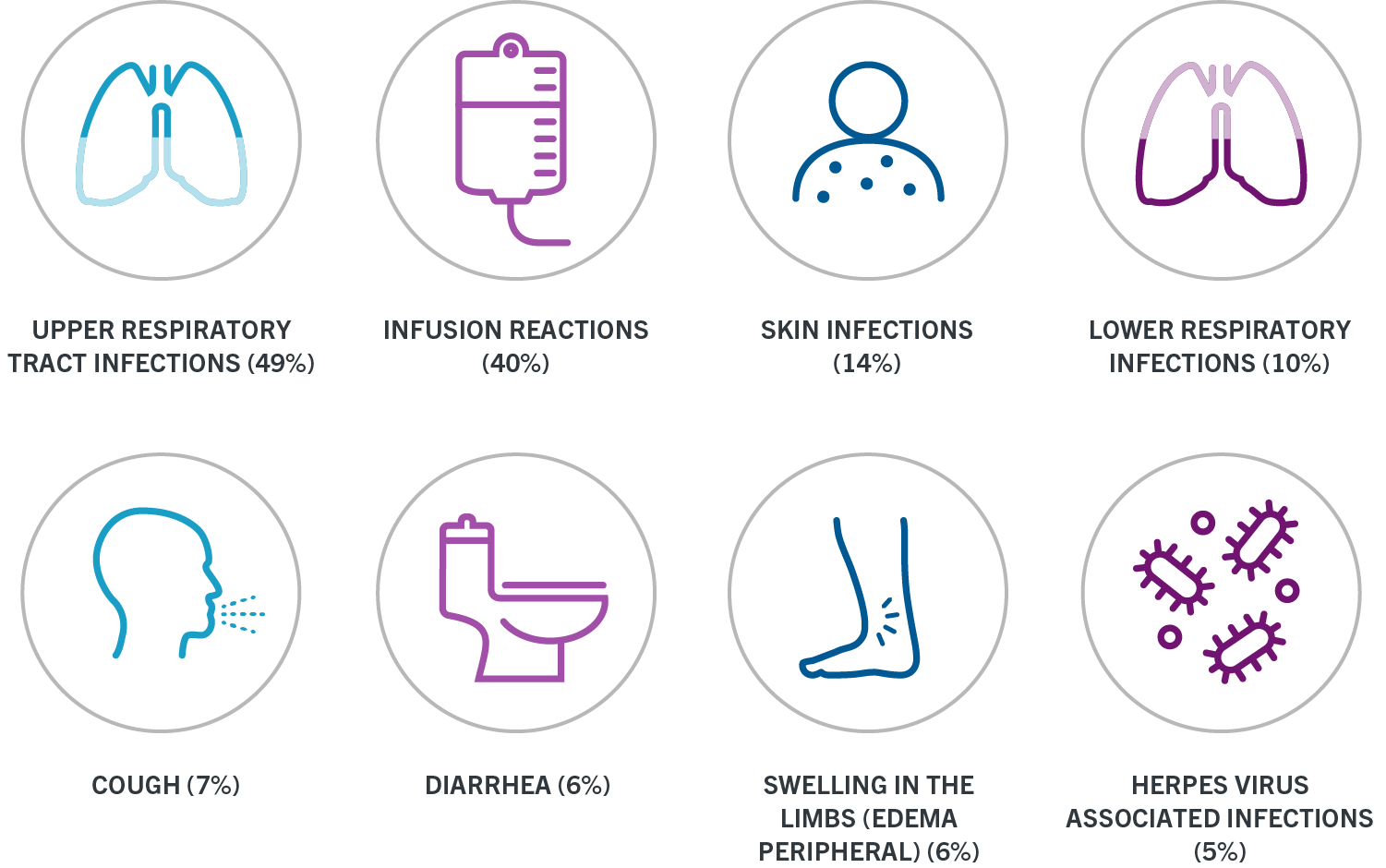
. Prescription Enrollment Form. Swelling of the throat. RMS and PPMS and their open-label extensions up to.
Ocrevus ocrelizumab Fax completed form to 8883021028. View full prescribing information and Boxed Warning. Have the prescribing physician complete the Physician Information sections.
It must be completed by the provider. Ad Learn More About OCREVUS As A Treatment Option That May Be Right For You. Prior Authorization Form for.
Ad Get patients started with AUBAGIO. The form includes patient insurance and prescription information. Ad Get patients started with AUBAGIO.
Is this a new start or continuation of therapy. View full prescribing information and Boxed Warning. Patients first name.
Content updated daily for ocrevus start form. Ad Get Started With KESIMPTA. Each vial contains 300 mg10 mL of OCREVUS for intravenous infusion.
Send all pages of the completed. Visit Official Patient Site for Information on a Treatment. Ad Learn More About OCREVUS As A Treatment Option That May Be Right For You.
300 mg250 ml NS IV on day 1. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary. Access Solutions is committed to helping your patients access the Genentech medicines they need providing assistance to your patients after OCREVUS is prescribed.
Complete Patient Information sections. Ocrevus ocrelizumab Vials are diluted in NS Subsequent doses one infusion 300mg10mL SDV to a final concentration of 12mgmL Every 6 months infuse 600mg in 500mL of 09 NS. If your patient has already begun treatment with drug samples of Ocrevus please choose new start of therapy.
These infusion reactions can happen for up to 24 hours after your infusion. Swelling of the throat. Ocrevus ocrelizumab injection is a preservative-free sterile clear or slightly opalescent and colorless to pale brown solution supplied as a carton containing one 300.
The OCREVUS Start Form is required for enrollment in OCREVUS Access Solutions. Once youve prescribed OCREVUS enroll your patients in OCREVUS Access Solutions. OCREVUS is a prescription medicine used to treat.
It is important that.

Ocrevus Ocrelizumab Ms Infusion Experience

Ocrevus Ocrelizumab In Ms Uses Side Effects And More Multiple Sclerosis News Today

What Is Multiple Sclerosis Ms Symptoms Causes Diagnosis Treatment And Prevention Everyday Health

Ocrevus Ocrelizumab Ms Infusion Experience

Multiple Sclerosis Ms Awareness Month 2022 Everyday Health

Ocrevus Ocrelizumab Ms Infusion Experience

Ocrevus Ocrelizumab In Ms Uses Side Effects And More Multiple Sclerosis News Today

Ocrevus Ocrelizumab In Ms Uses Side Effects And More Multiple Sclerosis News Today

Ocrevus Ocrelizumab In Ms Uses Side Effects And More Multiple Sclerosis News Today

Multiple Sclerosis Ms News Research Studies Ms Research Articles
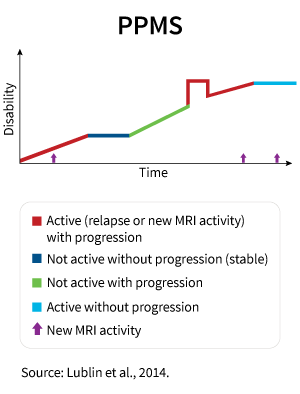
Primary Progressive Ms Ppms National Multiple Sclerosis Society

Ocrevus Ocrelizumab In Ms Uses Side Effects And More Multiple Sclerosis News Today

Ocrevus Ocrelizumab Ms Infusion Experience

Ocrevus Side Effects Cost Uses And More

Ocrevus Ocrelizumab Ms Infusion Experience

Multiple Sclerosis Symptoms Causes Diagnosis And Treatment